The Ancient Protein Clock That Ticks Without DNA

TL;DR: By 2030, wearable biosensors embedded in clothing could continuously monitor disease markers in your sweat, eliminating blood tests for many conditions. While devices are reaching FDA clearance today, accuracy challenges and privacy concerns remain as this technology transforms healthcare from episodic testing to continuous health surveillance.
By 2030, checking your blood sugar might be as simple as wearing a T-shirt. No finger pricks, no blood draws, no clinic visits - just a sensor embedded in fabric that continuously reads your sweat. This isn't science fiction anymore. Researchers have already demonstrated sweat glucose sensors with over 95% accuracy, and the FDA has started clearing wearable biosensors for patient monitoring. The question isn't whether this technology will arrive, but how fast we'll adapt once it does.
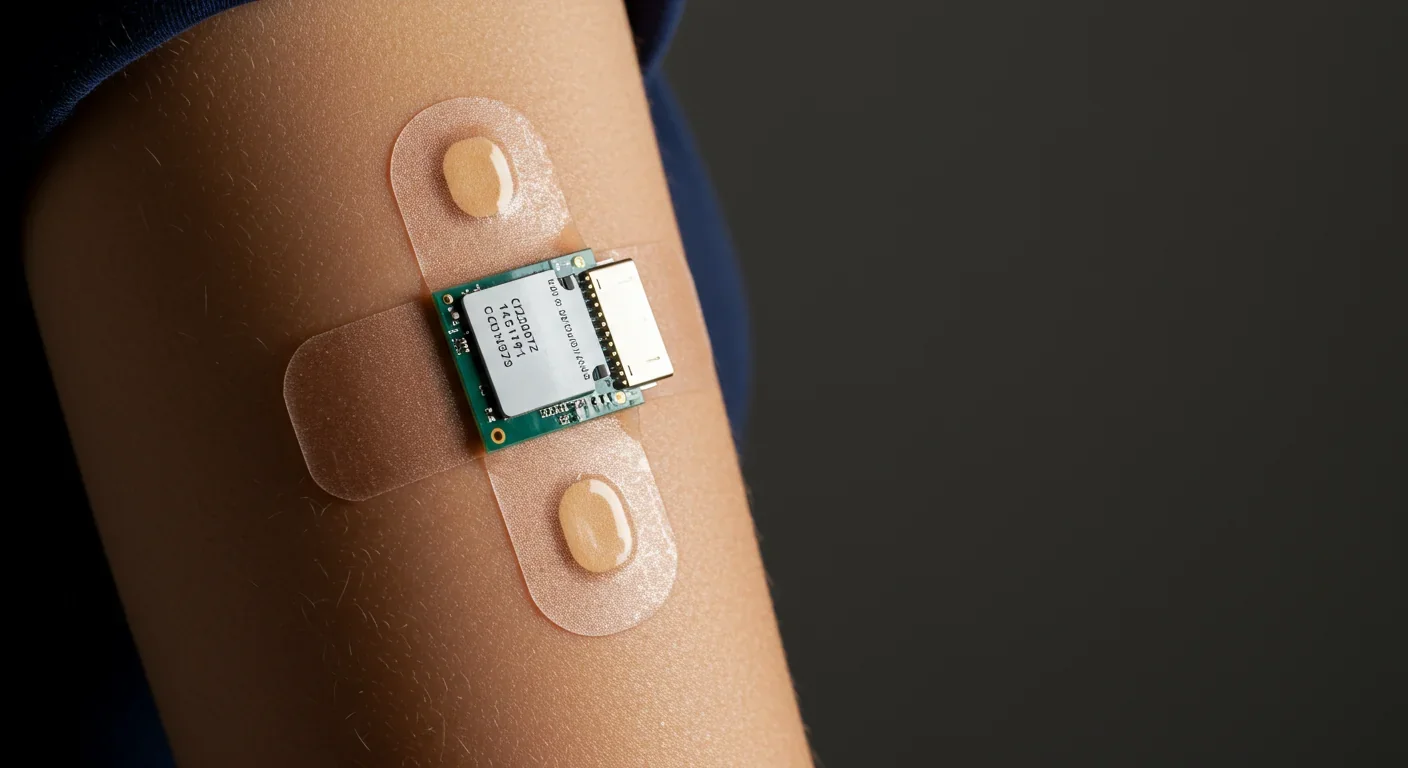
For decades, medical diagnostics meant invasive procedures. Blood tests required needles. Glucose monitoring meant pricking your finger multiple times daily. Even basic health tracking demanded trips to the doctor's office. But wearable biosensors are flipping that model, transforming disease detection from episodic snapshots into continuous, real-time monitoring through non-invasive analysis of bodily fluids like sweat, tears, and saliva.
Your sweat contains far more information than you realize. Beyond just water and salt, sweat harbors glucose, lactate, cortisol, uric acid, inflammatory cytokines, and dozens of other biomarkers that reflect your metabolic state, stress levels, hydration, and even early disease processes.
The technological leap enabling this revolution combines three key advances: flexible bioelectronics that conform to skin without restricting movement, electrochemical sensors sensitive enough to detect biomarkers at picomolar concentrations, and machine learning algorithms that translate sweat chemistry into clinically meaningful insights.
Penn State researchers developed a graphene-foam glucose sensor woven into fabric that achieved 91% sensitivity over three weeks of continuous wear while simultaneously monitoring body temperature and pH levels. The sensor uses laser-induced graphene foam - essentially a sponge-like carbon structure - to catalyze electrochemical reactions when glucose molecules from sweat contact its surface. The resulting electrical current correlates with glucose concentration, and a personalized algorithm corrects for individual differences in sweat rate and skin properties.

Sweat glucose concentrations are 10-20 times lower than blood glucose, creating a sensitivity challenge that requires both advanced materials and personalized algorithms to overcome.
This matters because current continuous glucose monitors use microneedle patches that penetrate skin to sample interstitial fluid. They work well - representing the gold standard for diabetes management - but they're invasive, require replacement every 14 days, and cost patients hundreds of dollars monthly. A textile sensor you simply wear and wash changes the economics and user experience entirely.
The diagnostic capabilities of wearable biosensors extend far beyond diabetes, though glucose monitoring remains the most developed application.
Metabolic disorders: Beyond glucose, sweat sensors detect lactate (indicating muscle fatigue and metabolic stress), uric acid (linked to gout and kidney function), and branched-chain amino acids (markers of protein metabolism). The Epicore Biosystems ECHO Smart Patch, already deployed in sports medicine, measures pH, glucose, and lactate to provide insights into hydration and metabolic status during training.
Cardiovascular monitoring: LifeSignals' UbiqVue 2A system, which received FDA 510(k) clearance in 2024, continuously monitors SpO₂ (blood oxygen saturation), two-channel ECG, pulse rate, respiration rate, body temperature, and motion - twelve parameters total - from a single chest-worn biosensor. The device addresses a critical need for remote patient monitoring in heart failure and post-surgical care, transmitting encrypted data in real-time to clinicians who can receive alerts before a patient's condition deteriorates.
Stress and hormone tracking: Cortisol, the body's primary stress hormone, appears in sweat at detectable concentrations. Researchers have demonstrated graphene-based sensors capable of detecting cortisol at picomolar levels, potentially enabling continuous stress monitoring for mental health applications.
Cancer screening: This remains more speculative, but promising. Tumor-derived exosomes - nanoscale vesicles that cancer cells release into bodily fluids including tears and saliva - carry tumor-specific proteins and RNA. Electrochemical biosensors can detect these exosomes through selective antibody binding, opening pathways for non-invasive liquid biopsies. The technology is still in research phases, not yet approaching clinical use, but several academic groups are actively pursuing tear-based cancer screening.

Sleep disorders: PranaQ's TipTraQ device, cleared by the FDA in early 2025, uses tri-wavelength photoplethysmography (PPG) and motion sensors to diagnose sleep apnea at home with clinical-grade accuracy, validated against polysomnography at Duke University Hospital and UCSF. The device measures SpO₂, pulse rate, blood pressure trends, snoring, and respiration patterns - replacing a lab-based sleep study that typically costs thousands of dollars.
Here's where the technology gets complicated. The accuracy of wearable biosensors depends heavily on which biomarker you're measuring and which body fluid you're sampling.
The gold standard comparison: Traditional blood tests remain the clinical benchmark. Blood glucose monitors achieve accuracy within 5-10% of laboratory reference values. For a biosensor to replace blood testing, it needs to match or approach that standard.
Sweat glucose: the challenge: Glucose concentrations in sweat range from 0.02 to 0.6 millimolar (mM), roughly 10-20 times lower than blood glucose (typically 4-10 mM). This creates a sensitivity problem - sensors must detect much smaller amounts. Additionally, sweat glucose doesn't correlate perfectly with blood glucose at every moment; the relationship varies with sweat rate, skin temperature, and time lag between blood and sweat equilibration.
Joseph Wang's team at UC San Diego solved this with personalized algorithms. Their touch-based sweat sensor achieved over 95% accuracy predicting blood glucose before and after meals, but only after developing individual calibration models for each user. Calibration requires a finger-stick blood test just once or twice monthly - dramatically better than multiple daily pricks, but still necessary.
"Smart textiles are still in early development and not yet as precise as current glucose monitors, but they represent an exciting step toward more seamless, noninvasive monitoring."
- Dr. Disha Narang, endocrinologist
Tear glucose: the failure case: Google (through its Verily division) famously pursued smart contact lenses for glucose monitoring from 2014-2018, developing prototypes that generated readings once per second. They discontinued the project because tear glucose showed weak correlation with blood glucose - the biochemical relationship simply wasn't reliable enough to meet accuracy requirements for diabetes management. Endocrinologists who reviewed the data expressed skepticism from the start.
This failure teaches a crucial lesson: not all bodily fluids are equal for biosensing. The physiological relationship between blood chemistry and tear chemistry differs fundamentally from the blood-sweat relationship.
SpO₂ and vital signs: proven accuracy: For parameters like oxygen saturation, heart rate, and respiration, the accuracy story is much better. LifeSignals' chest-worn biosensor explicitly addressed SpO₂ accuracy across diverse skin tones (a known limitation of standard pulse oximeters that can mis-read darker skin), meeting FDA requirements for clinical deployment. These sensors don't rely on sweat chemistry - they use optical and electrical measurements similar to hospital monitors.

The wearable biosensor market sits at an interesting inflection point - several products have reached commercialization, but mass consumer adoption remains limited.
Already on the market:
Abbott's FreeStyle Libre, which samples interstitial fluid through a microneedle patch, has become the most successful continuous glucose monitor globally, with millions of users. It's FDA-cleared, covered by many insurance plans, and provides real-time glucose data to a smartphone app. But it's not sweat-based - it still requires a needle insertion every 14 days.
LifeSignals UbiqVue 2A, cleared by the FDA in late 2024, is a single-use chest biosensor for continuous multi-parameter vital sign monitoring in home, remote, and hospital settings. It's aimed at clinical use rather than consumer wellness - hospitals and remote monitoring programs are the target customers.
Epicore Biosystems' ECHO Smart Patch measures sweat biomarkers for hydration and metabolic status, primarily marketed to athletes and military personnel. It's commercially available but positioned as a performance optimization tool rather than medical diagnostic.
In development but not yet cleared:
Smart textile glucose sensors, like the graphene-foam garments from Penn State, remain in research trials. Dr. Disha Narang notes these are "still in early development and not yet as precise as current glucose monitors," though a pilot study could lead to FDA submission within a year.
Tear-based sensors beyond the failed Google lens continue in academic research but face the fundamental biochemical correlation problem.
The pattern is clear: sensors measuring well-established physiological signals (heart rate, oxygen saturation, temperature) are reaching market first, while those attempting to extract novel diagnostic information from sweat chemistry are still working through accuracy and reliability challenges.
Despite impressive laboratory results, multiple technical barriers slow the path from prototype to widespread clinical use.
Biomarker correlation and calibration: As the Google lens failure demonstrated, you can't assume that a biomarker in sweat or tears perfectly mirrors its concentration in blood. The relationship is often nonlinear, time-lagged, and influenced by factors like sweat rate, skin temperature, and recent food intake. Personalized algorithms help, but they add complexity and typically require periodic blood-test calibration.
Technical barriers remain substantial: sensor stability degrades with exposure to sweat and skin, variable sweat production complicates sampling, and battery life limits continuous operation. These aren't trivial engineering problems - they're why most current devices are either single-use or require frequent replacement.
Sensor stability and longevity: A sensor exposed to sweat faces a hostile environment - salt, proteins, dead skin cells, and bacteria all contribute to biofouling, gradually degrading sensor performance. Dr. Narang identifies "sensor durability with repeated sweat and washing, and the risk of data drift as sensors degrade" as primary safety concerns. Enzyme-based sensors are particularly vulnerable since the enzymes denature over time.
This explains why current products are either single-use disposable (UbiqVue 2A) or require frequent replacement (FreeStyle Libre every 14 days). Developing sensors that maintain accuracy for months remains an unsolved engineering problem.
Variable sweat production: You don't sweat uniformly throughout the day. At rest, sweat production may be minimal, providing insufficient sample for reliable measurements. Advanced microfluidic designs - including capillary-bursting valves, Tesla structures, and iontophoretic stimulation (using small electrical currents to induce localized sweating) - attempt to guarantee reliable sweat collection across rest and exercise, but these add complexity and power requirements.
Power and connectivity: Continuous sensing requires continuous power. Battery life becomes a limiting factor, especially for devices transmitting data wirelessly. Some research groups are exploring kinetic energy harvesting (converting body motion into electricity) and ultra-low-power circuitry, but these technologies aren't yet mature. Most current devices require charging every few days or use single-use batteries.
Data security and wireless reliability: Medical data flowing continuously from body-worn sensors creates new privacy vulnerabilities. LifeSignals addressed this by encrypting data before transmission and meeting latency requirements for timely clinical alerts, but these considerations add engineering complexity. A biosensor that loses wireless connection during a critical health event could have serious consequences.
Skin irritation and comfort: Wearing sensors 24/7 means prolonged skin contact with adhesives, electrodes, and synthetic materials. Skin irritation, allergic reactions, and simple discomfort limit user adherence. Flexible bioelectronics that physically conform to skin improve signal fidelity by minimizing motion artifacts while enhancing comfort - materials science advances in stretchable electronics, breathable substrates, and hypoallergenic adhesives are as important as the sensing chemistry itself.
Continuous biosensing generates unprecedented volumes of intimate health data, raising thorny questions about ownership, access, and protection.
The data stream is revealing: Unlike a once-yearly blood test, a wearable biosensor captures your physiological state minute-by-minute. That granular data reveals far more than just glucose levels - it can show sleep patterns, stress responses, sexual activity, alcohol or drug use, and potentially even early signs of pregnancy or illness before you're aware. Insurance companies, employers, law enforcement, and marketers would all find such data valuable.
Current regulatory frameworks are inadequate: In the US, HIPAA protects health information held by healthcare providers and insurers, but it doesn't cover data collected by consumer wellness devices. If your smartwatch collects biosensor data, the manufacturer may legally sell that information to third parties unless you carefully review privacy settings. The FDA regulates medical devices but doesn't directly govern data privacy.
"The FDA clearance underscores our commitment to delivering scalable solutions. We addressed SpO₂ accuracy across diverse skin tones, ensured data security, maintained wireless reliability, and met latency requirements for timely alert generation."
- Saravanan Balasubramanian, VP of Medical Technology, LifeSignals
The security challenge: Wirelessly transmitted health data creates attack surfaces for hackers. Encrypted transmission helps, but vulnerabilities in smartphone apps, cloud storage, or the sensors themselves could expose sensitive information. A compromised biosensor could potentially be manipulated to deliver false readings, with life-threatening consequences for someone making treatment decisions based on the data.
Consent and secondary use: Many users accept long, complex terms of service without fully understanding how their data might be used. If a biosensor detects early cancer markers, does the manufacturer have an ethical obligation to alert you even if you didn't purchase the "cancer screening" feature? Can your health data be subpoenaed in criminal cases? These legal and ethical frameworks are still forming.
The path forward likely requires updated legislation specifically addressing continuous biosensing data, stronger default privacy protections, and transparent user control over data sharing.
The shift from episodic testing to continuous monitoring could fundamentally reshape healthcare delivery and economics.
Earlier disease detection: Continuous monitoring enables earlier disease detection than periodic clinic-based testing. A biosensor could detect metabolic changes hours or days before symptoms appear, enabling intervention at earlier, more treatable stages. For conditions like sepsis, diabetic ketoacidosis, or heart failure decompensation, those extra hours can mean the difference between outpatient management and ICU admission.
Hospital-to-home shift: Remote patient monitoring using biosensors allows hospitals to discharge patients earlier while maintaining clinical oversight. A heart failure patient wearing a UbiqVue sensor at home provides continuous data to their care team, who can detect deterioration and intervene before an emergency room visit becomes necessary. This reduces costly hospitalizations while improving patient quality of life.
Medicare and private insurers are starting to reimburse remote monitoring services, recognizing the cost savings. LifeSignals explicitly positions their single-use biosensor as enabling "population health management" at scale - monitoring thousands of high-risk patients simultaneously at a fraction of the cost of traditional care.
Personalized medicine: Continuous data enables n-of-1 trials - rigorous experiments where individuals test how their body responds to specific interventions. A diabetes patient could systematically determine which foods spike their glucose, which exercise routines optimize their control, and how much sleep they need for metabolic health. AI-driven predictive algorithms can learn individual patterns and provide personalized glycemic predictions and early warnings.
The cost equation: Current continuous glucose monitors cost $200-400 monthly in the US without insurance. Wearable textile sensors could dramatically reduce costs since they use inexpensive screen-printed electrodes and standard fabrics rather than specialized microneedle patches. If a reusable sensor shirt costs $50 and lasts three months, the economics favor widespread adoption.
However, the full cost includes not just the sensor but also the cloud infrastructure, data analysis, and clinical interpretation. Healthcare systems will need to develop workflows for triaging alerts from thousands of sensors, determining which findings require immediate attention, and avoiding alarm fatigue.
Unequal access risk: As with many health technologies, there's a danger that wearable biosensors primarily benefit wealthy, health-conscious consumers with good insurance while remaining out of reach for underserved populations. Regulatory barriers in Europe, including the stringent MDR 645/2017 requirements that took effect in 2021, may slow innovation and increase costs, potentially limiting access.
Where does this technology go from here, and how soon?
Multimodal integration: The next generation of biosensors won't just measure one biomarker but will combine multiple signals - glucose, lactate, cortisol, inflammatory markers, vital signs - into a holistic metabolic assessment. Integrating biochemical markers with physiological signals provides far richer information than either alone. A sensor that detects elevated cortisol plus disrupted sleep plus rising inflammatory cytokines could flag burnout or depression risk before a mental health crisis occurs.
Closed-loop therapeutic systems: Imagine a sensor that not only detects low blood sugar but triggers an insulin pump to correct it, or a patch that delivers medication automatically when inflammatory markers spike. These closed-loop systems are already in development, combining biosensing with transdermal drug delivery through microneedles or iontophoresis.
Advanced manufacturing: Three-dimensional printing technologies enable fabrication of disposable, paper-based sweat sensors that could be cost-effective and environmentally sustainable at scale. Custom-printed sensors tailored to individual body shapes and specific biomarker panels could become economically viable.
Cancer screening: While still speculative, tear-based exosome detection for cancer screening represents a high-value target. If technical challenges around sensitivity and specificity can be solved, a daily-wear sensor that alerts to early tumor markers could catch cancers at highly curable stages. Multiple research groups are pursuing this, but realistic clinical deployment is probably 5-10 years away, not imminent.
Realistic timeline for mainstream adoption:
Now to 2027: FDA-cleared sensors for specific clinical indications (diabetes, sleep apnea, post-surgical monitoring) expand in hospitals and specialized clinics. Early-adopter consumers experiment with wellness-focused devices, but accuracy and reliability issues limit mass market penetration.
2027-2030: Improved sensor stability and calibration algorithms enable truly non-invasive sweat glucose monitoring accurate enough to reduce or eliminate finger-sticks for many diabetes patients. Smart textile sensors receive FDA clearance and begin gradual market entry. Insurance reimbursement for remote monitoring expands, accelerating adoption in chronic disease management.
2030-2035: Multimodal biosensors integrating multiple biomarkers become standard for preventive health monitoring in higher-income countries. Integration with AI health assistants enables proactive health management. Regulatory frameworks and data privacy laws mature to address the unique challenges of continuous biosensing.
Beyond 2035: Truly comprehensive biosensing - tracking dozens of biomarkers continuously, predicting disease risk years in advance, enabling precision medicine at population scale - becomes technically feasible. The social, economic, and ethical implications at that point become profound.
The trajectory of wearable biosensors mirrors the early smartphone era in instructive ways.
In 2007, when Apple launched the first iPhone, many experts dismissed it as an expensive toy. Established players like Nokia and BlackBerry dominated the market with devices optimized for their core functions - calling and email. The idea that most people would pay $500 for a device that combined mediocre phone quality, battery-hungry web browsing, and apps of uncertain value seemed economically dubious.
Yet within five years, smartphones had transformed communication, commerce, navigation, photography, and daily life in ways that seemed impossible beforehand. The shift happened not because the iPhone perfected any single function, but because it integrated multiple capabilities in a form factor you already carried, creating emergent value that exceeded the sum of its parts.
Wearable biosensors face similar skepticism today. They don't yet match the gold-standard accuracy of laboratory tests. They require periodic calibration. They're expensive and not covered by most insurance. Critics reasonably ask: why not just get a blood test when needed?
But that question misses the transformative potential of continuous data. A quarterly blood test tells you your glucose at one moment. A continuous biosensor shows how your body responds to specific foods, stress, sleep patterns, exercise timing, and medications - information simply not available from episodic testing. That shift from snapshots to movies fundamentally changes what's medically possible.
The smartphone parallel suggests that widespread adoption will happen when biosensors integrate seamlessly into products people already use - clothing, watches, jewelry - rather than requiring dedicated medical devices. When monitoring becomes invisible and automatic rather than deliberate and inconvenient, behavior changes.
How quickly wearable biosensors spread globally depends heavily on local healthcare systems, regulatory environments, and economic structures.
United States: market-driven innovation with unequal access: The FDA's 510(k) clearance pathway has enabled relatively rapid approval of novel biosensors, with multiple devices reaching market in 2024-2025. Strong venture capital funding and partnerships between medtech startups and established healthcare companies accelerate innovation. However, fragmented insurance coverage means access depends heavily on employment status and plan quality, likely creating a two-tier system where affluent consumers benefit first.
Europe: stringent regulation slowing commercialization: The MDR 645/2017 regulations that took full effect in 2021 require substantially more clinical validation and post-market surveillance than previous directives, increasing both costs and time-to-market. Patent activity for glucose biosensors dropped sharply after 2021, suggesting that European regulatory burdens are discouraging innovation in this space. Once approved, however, broad public health insurance coverage could enable equitable access across socioeconomic groups.
Asia: diverse strategies reflecting different priorities: Japan's aging population and advanced manufacturing base create strong incentives for remote monitoring technologies that reduce healthcare labor costs. China has aggressively pursued bioelectronics research and filed numerous patents, positioning domestic companies to compete globally. India faces different challenges - how to deploy biosensors at scale in settings without reliable electricity, internet connectivity, or cold-chain logistics, suggesting that ultra-low-cost, minimal-infrastructure solutions might emerge from that context.
Low-resource settings: leapfrogging potential: Just as mobile phones enabled many developing countries to skip landline infrastructure, disposable biosensors manufactured locally via 3D printing technologies could enable diagnostic capabilities in areas that never built extensive laboratory networks. The key is whether designs can be simplified and localized rather than requiring complex supply chains and technical support.
As wearable biosensors transition from experimental to mainstream, several practical considerations matter for individuals:
Understand what the technology can and can't do: Current biosensors excel at continuous tracking of established physiological parameters but shouldn't replace comprehensive medical evaluation for serious symptoms. A glucose sensor won't diagnose why you're losing weight or feeling fatigued - it provides one data stream that requires interpretation in clinical context.
Ask about accuracy and calibration: If your doctor recommends a biosensor, ask: What accuracy studies support this device? How often does it need calibration? What's the false positive and false negative rate? These aren't academic questions - you might make treatment decisions based on this data.
Protect your data: Before using a consumer biosensor, read the privacy policy carefully. Opt out of data sharing where possible. Use strong passwords and enable two-factor authentication on associated apps. Recognize that once your health data enters commercial databases, you may lose control over how it's used.
Demand insurance coverage: As biosensors demonstrate cost savings through earlier intervention and reduced hospitalizations, insurance companies should cover them like other preventive technologies. Advocating for coverage, both individually and through patient organizations, helps accelerate equitable access.
Stay informed about regulatory developments: Laws governing biosensor data ownership, privacy, and usage are actively being written. Engage with these policy discussions rather than accepting whatever framework emerges by default. Your digital health rights are being defined right now.
The skills to develop: health data literacy (interpreting trends rather than fixating on individual readings), digital security practices, and the ability to have informed conversations with healthcare providers about what biosensor data means for your specific situation.
The implications of widespread biosensor adoption extend far beyond individual health management.
Epidemiology in real time: Public health surveillance currently relies on voluntary reporting and laboratory testing with substantial time lags. Imagine instead that millions of biosensors detect the early signature of an emerging infectious disease - distinctive inflammatory marker patterns before symptoms appear. Public health officials could identify outbreak epicenters within hours rather than weeks, enabling much faster intervention.
Pharmaceutical development: Clinical trials could monitor drug effects continuously rather than at scheduled visits, capturing side effects and efficacy signals that episodic testing misses. This could accelerate drug development while improving safety.
Performance optimization: Athletes already use biosensors to optimize training and recovery, but the technology could extend to any performance domain. Firefighters and soldiers could receive alerts when physiological stress reaches dangerous levels. Knowledge workers could track how sleep, nutrition, and work patterns affect cognitive performance.
Social equity questions: Will employers require biosensor monitoring as a condition of employment, particularly in safety-critical industries? Could insurance premiums be tied to biosensor data? If continuous monitoring can predict disease risk years in advance, does that create a new form of discrimination against the "pre-sick"? We're designing these technologies faster than we're answering these ethical questions.
The quantified-self paradox: Complete health data could empower informed decision-making, or it could fuel anxiety and hypochondria. There's evidence that excessive health monitoring correlates with increased anxiety in some individuals. The challenge is designing systems that inform without overwhelming, that provide agency without creating obligatory self-surveillance.
Wearable biosensors represent genuine paradigm shifts in disease detection and health management. The technology is real, not hype. Devices are reaching market. Evidence of clinical utility is accumulating. The FDA is clearing new systems at an accelerating pace.
But let's be clear about where we actually are: most applications remain in research or early commercialization. The accuracy and reliability challenges are substantial. The regulatory, economic, and ethical frameworks to support widespread deployment are still forming. Mass consumer adoption is years away, not months.
For people managing chronic conditions like diabetes, current biosensors already offer meaningful improvements over traditional monitoring - fewer finger pricks, continuous data, better control. For remote patient monitoring in cardiology and post-surgical care, the business case is strong and adoption is accelerating.
For broader preventive health monitoring in the general population, we're still in the early adopter phase. The technology isn't yet accurate, convenient, or affordable enough for most people to justify adding another device to their lives.
But that's changing, and probably faster than you expect. The research pipeline is robust. Commercial products are proliferating. Regulatory pathways are becoming clearer. Insurance reimbursement is expanding. Manufacturing costs are dropping as production scales.
By 2030, checking your health metrics by simply living your life - wearing normal clothes, sleeping, working - could be as unremarkable as checking your phone for messages is today. The question isn't whether that future arrives, but whether we build the safeguards and social structures to ensure it improves human flourishing rather than creating new forms of surveillance and inequality.
The biosensors are coming. How we deploy them, who has access, what we do with the data, and what rights individuals retain over their own biological information - those decisions are still ours to make.

Ahuna Mons on dwarf planet Ceres is the solar system's only confirmed cryovolcano in the asteroid belt - a mountain made of ice and salt that erupted relatively recently. The discovery reveals that small worlds can retain subsurface oceans and geological activity far longer than expected, expanding the range of potentially habitable environments in our solar system.

Scientists discovered 24-hour protein rhythms in cells without DNA, revealing an ancient timekeeping mechanism that predates gene-based clocks by billions of years and exists across all life.

3D-printed coral reefs are being engineered with precise surface textures, material chemistry, and geometric complexity to optimize coral larvae settlement. While early projects show promise - with some designs achieving 80x higher settlement rates - scalability, cost, and the overriding challenge of climate change remain critical obstacles.

The minimal group paradigm shows humans discriminate based on meaningless group labels - like coin flips or shirt colors - revealing that tribalism is hardwired into our brains. Understanding this automatic bias is the first step toward managing it.

In 1977, scientists discovered thriving ecosystems around underwater volcanic vents powered by chemistry, not sunlight. These alien worlds host bizarre creatures and heat-loving microbes, revolutionizing our understanding of where life can exist on Earth and beyond.

Automated systems in housing - mortgage lending, tenant screening, appraisals, and insurance - systematically discriminate against communities of color by using proxy variables like ZIP codes and credit scores that encode historical racism. While the Fair Housing Act outlawed explicit redlining decades ago, machine learning models trained on biased data reproduce the same patterns at scale. Solutions exist - algorithmic auditing, fairness-aware design, regulatory reform - but require prioritizing equ...

Cache coherence protocols like MESI and MOESI coordinate billions of operations per second to ensure data consistency across multi-core processors. Understanding these invisible hardware mechanisms helps developers write faster parallel code and avoid performance pitfalls.