The Ancient Protein Clock That Ticks Without DNA

TL;DR: Extracellular vesicles are nano-sized cellular messengers that can both spread cancer and heal tissue. Research is turning these biological couriers into diagnostic tools and therapeutic delivery systems.

Within a decade, your annual checkup might skip the needle entirely. Instead of blood tests, a simple saliva swab could reveal whether cancer is silently spreading through your body. That's not science fiction - it's the emerging reality of extracellular vesicle research, a field that's transforming how we detect disease and deliver treatments at the cellular level.
These nano-sized bubbles, released by every cell in your body, carry molecular cargo that can either fuel cancer's spread or orchestrate healing. Understanding their dual nature could revolutionize everything from early cancer detection to regenerative medicine.
Extracellular vesicles are membrane-wrapped packages roughly 30 to 1,000 nanometers across - so small that thousands could fit inside a single human cell. Every cell type manufactures these carriers, loading them with proteins, lipids, and genetic material before shipping them to neighboring or distant cells.
Think of EVs as biological USB drives. They don't just transmit simple on-off signals like hormones. Instead, they deliver complex datasets - entire software updates for recipient cells. A cancer cell can package instructions for drug resistance into an EV, send it across the body, and reprogram a healthy cell to ignore chemotherapy before the tumor cells even arrive.
The sophistication of this system lies in cargo selection. Cells don't randomly stuff molecules into EVs. Specific proteins act as gatekeepers, determining which RNAs, proteins, and lipids get packaged. RNA-binding proteins recognize sequence motifs in microRNAs, essentially reading molecular barcodes that grant passage into vesicles.
This selective loading process explains why EVs from cancer cells carry such different payloads than those from healthy stem cells. The contents determine the message, and the message determines whether the recipient cell becomes an ally or an enemy.
When cancer spreads, it doesn't just randomly throw cells into the bloodstream and hope they survive. It sends advance scouts - millions of EVs that prepare distant organs to welcome tumor cells.
This process, called pre-metastatic niche formation, begins months before metastasis becomes detectable. Tumor-derived EVs travel through circulation to organs like the liver, lungs, or bones. There, they reprogram the local environment: suppressing immune cells that would attack invaders, stimulating blood vessel growth to provide nutrients, and remodeling tissue architecture to create landing pads.
Research shows that EVs containing VEGF protein drive tumor blood vessel growth while simultaneously protecting that VEGF from bevacizumab, a common anti-angiogenic drug. The heat shock protein Hsp90 in these vesicles acts like a molecular bodyguard, physically shielding VEGF from antibody therapy.
Even more troubling, cancer cells use EVs to transfer drug resistance between tumor cells. When chemotherapy kills 99% of cancer cells, the surviving 1% can share their resistance mechanisms via EVs, essentially tutoring their neighbors in survival strategies. It's peer-to-peer file sharing for staying alive under pharmaceutical assault.
The immune evasion tactics are equally sophisticated. Tumor EVs carry PD-L1, the same checkpoint protein that tricks T cells into standing down. They deliver this immunosuppressive signal to both immune cells and stromal cells throughout the body, creating an environment where cancer cells operate with impunity.
The same delivery system cancer exploits for destruction, our own regenerative cells use for repair. Mesenchymal stem cells, which reside in bone marrow and other tissues, constantly release EVs loaded with anti-inflammatory signals and growth factors.
When tissue gets damaged - whether from injury, disease, or aging - these stem cell-derived EVs rush to the scene. They reduce inflammation, stimulate tissue regeneration, and coordinate the complex cellular choreography of healing. Unlike stem cell transplants, which carry risks of immune rejection and uncontrolled growth, EVs deliver therapeutic effects without bringing whole cells.
In cardiac regeneration, mesenchymal stem cell EVs have shown remarkable potential. They promote blood vessel formation in damaged heart tissue, reduce scarring, and help surviving cardiac muscle cells recover function. Early clinical trials are testing whether injecting these EVs after heart attacks can limit damage and improve long-term outcomes.
The immunomodulatory properties extend beyond physical injuries. MSC-derived EVs are being investigated for autoimmune and inflammatory diseases, where the goal is to calm overactive immune responses rather than stimulate them. The same cellular postal service that spreads cancer can also deliver peace treaties to warring immune cells.
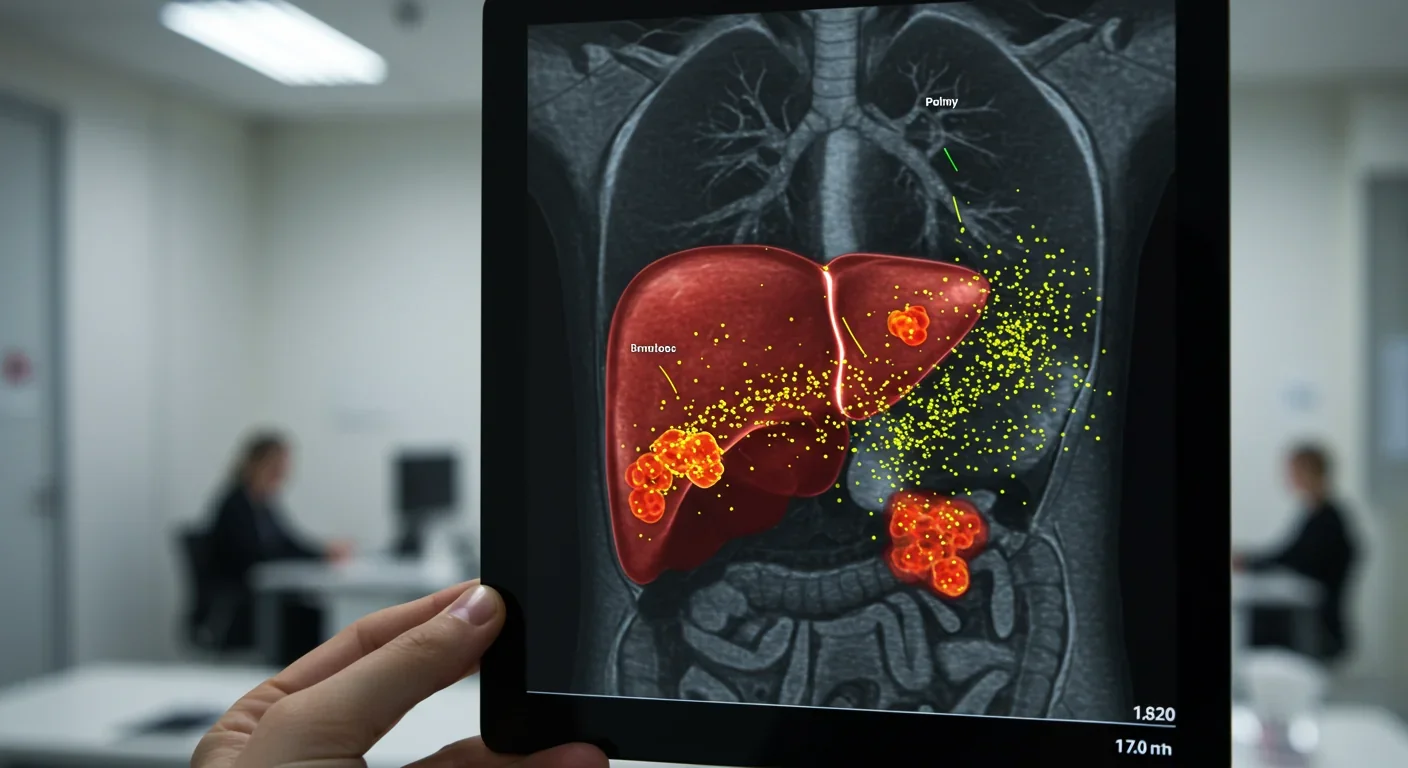
The diagnostic potential of EVs stems from a simple fact: they're everywhere. Blood, urine, saliva, cerebrospinal fluid - every bodily fluid contains EVs that reflect the state of their cells of origin. More importantly, the molecular signatures in these vesicles can reveal disease months or years before symptoms appear.
Cancer EVs carry tumor-specific proteins and mutated DNA fragments. A liquid biopsy analyzing EVs from a blood sample can potentially detect pancreatic cancer when it's still confined to the pancreas - a stage when surgery is curative but traditional diagnostics usually miss the disease entirely.
The approach works because tumor cells shed EVs constantly, and those vesicles maintain their cargo integrity in circulation. Unlike free-floating DNA or proteins that degrade quickly, EVs protect their contents within lipid membranes. This stability makes them ideal biomarkers.
Multiple research groups are developing EV-based tests for specific cancers. Some focus on particular microRNA signatures that distinguish aggressive tumors from benign growths. Others look at protein panels that indicate whether cancer has spread beyond the primary site.
The technology faces challenges. Isolating pure EV populations from complex biological fluids remains technically difficult and expensive. EVs from different cell types look similar under a microscope, so distinguishing cancer EVs from the billions of normal EVs requires sophisticated molecular profiling.
But progress is rapid. Commercial platforms now automate EV isolation and analysis, bringing costs down and throughput up. Within five years, EV liquid biopsies could become routine screening tools, catching cancers when they're most treatable.
If natural EVs can ferry molecular cargo so effectively, why not repurpose them as drug delivery vehicles? That's exactly what researchers are doing, with results that surpass traditional pharmaceutical approaches.
The advantages are compelling. EVs naturally cross biological barriers that block synthetic nanoparticles. They evade immune clearance because they're decorated with "self" proteins that tell the body they belong. And they inherently target specific cell types based on their surface molecules.
Engineered exosomes can be loaded with chemotherapy drugs, gene-editing tools like CRISPR, or therapeutic RNAs. Researchers modify the surface proteins to enhance targeting - for instance, adding antibodies that recognize tumor markers ensures EVs concentrate in cancer tissue rather than healthy organs.
The engineering approaches vary. Some teams genetically modify producer cells to naturally load desired cargo into their EVs. Others use physical methods like electroporation or chemical permeabilization to force drugs into purified vesicles. Each strategy has trade-offs between efficiency, scalability, and cargo compatibility.
One particularly clever application involves using EVs to overcome the blood-brain barrier. This protective membrane shields the brain from toxins but also blocks most drugs. EVs derived from certain cell types naturally cross this barrier through receptor-mediated transcytosis. Loading these brain-permeable EVs with drugs could enable treatment of previously unreachable tumors and neurodegenerative diseases.
Clinical trials are underway. Early-phase studies are testing EV-based delivery of chemotherapy for pancreatic and lung cancers. Others are exploring EVs loaded with anti-inflammatory compounds for inflammatory bowel disease and rheumatoid arthritis.
The field hasn't solved every challenge. Large-scale EV production remains expensive and technically complex. Quality control is difficult when your product is biological and inherently variable. Regulatory agencies are still developing frameworks for approving EV-based therapeutics, which don't fit neatly into existing drug or biological product categories.
Here's the paradox keeping researchers awake at night: the same EVs you harness for therapy might also help the disease you're fighting.
Imagine treating a patient's cancer with drug-loaded EVs. Those therapeutic vesicles might destroy tumor cells effectively. But the dying cancer cells release their own EVs, potentially spreading resistance or pre-conditioning distant sites for metastasis. You're fighting fire with fire, but fire doesn't care whose side it's on.
This isn't theoretical. Studies show that some therapeutic interventions that generate cellular stress can actually increase EV production by target cells, including cancer cells. Chemotherapy and radiation - the standard treatments that kill cancer cells - might also trigger surviving tumor cells to release more immunosuppressive and pro-metastatic EVs.
The field is grappling with these complexities through combination strategies. Rather than using EVs alone, researchers are exploring:
EV inhibitors: Blocking cancer cells from producing or releasing EVs in the first place, cutting off their communication networks
EV decoys: Flooding the system with engineered vesicles that compete with tumor EVs for receptor binding but deliver inert or therapeutic cargo
Targeted destruction: Developing ways to specifically eliminate cancer-derived EVs from circulation while preserving beneficial ones
One promising approach uses antibody-based systems that recognize tumor-specific proteins on EV surfaces and tag them for immune clearance. It's like training border patrol to recognize and intercept specific smuggled goods while letting legitimate shipments through.

Five converging trends are accelerating EV research from laboratory curiosity to clinical reality.
First, isolation and analysis technologies are becoming standardized and affordable. What required expensive ultracentrifuges and days of labor now happens in automated microfluidic devices in hours. This democratization means smaller research groups can contribute insights, and clinical laboratories can realistically implement EV testing.
Second, artificial intelligence is solving the cargo prediction problem. Machine learning models trained on thousands of EV profiles can now predict which molecules will be packaged into EVs based on cell state and environmental conditions. This allows rational design of producer cells for therapeutic EVs rather than trial-and-error approaches.
Third, the regulatory landscape is crystallizing. Agencies like the FDA are engaging with researchers early to establish appropriate standards for EV-based products. This proactive dialogue prevents the situation where promising therapies languish because no clear approval pathway exists.
Fourth, manufacturing is scaling up. Companies are developing bioreactor systems that produce clinical-grade EVs in quantities sufficient for human trials. The cost per dose is dropping from thousands of dollars to hundreds - still expensive, but approaching feasibility for serious diseases.
Fifth, combination approaches are showing synergy. Using engineered EVs alongside checkpoint inhibitors or CAR-T cell therapy produces better results than either alone. The vesicles can suppress the immunosuppressive tumor microenvironment while checkpoint inhibitors remove the brakes from T cells, allowing the immune system to attack more effectively.
Within the next decade, EVs will likely touch your healthcare in multiple ways, whether you're healthy or ill.
For healthy individuals, annual EV screening could become as routine as cholesterol checks. A single blood draw analyzed for tumor-derived, inflammation-associated, and metabolic EVs would provide an early warning system for cancer, autoimmune conditions, and cardiovascular disease. Catching these conditions early transforms prognosis - pancreatic cancer detected at stage I has an 80% five-year survival rate compared to 3% when caught at stage IV.
For patients with chronic diseases, EV-based therapeutics offer hope where current treatments fail or cause intolerable side effects. Inflammatory bowel disease patients could receive EVs that calm intestinal inflammation without the infection risks of immunosuppressive drugs. Heart failure patients might get periodic infusions of cardiac-repair EVs that slowly regenerate damaged muscle.
Cancer treatment could become more personalized and less toxic. Instead of flooding the body with chemotherapy that kills healthy and cancerous cells alike, targeted EVs would deliver higher drug concentrations specifically to tumors while sparing normal tissue. Monitoring tumor-derived EVs during treatment would reveal resistance emergence weeks before tumors regrow, allowing therapeutic adjustments before disease progresses.
Regenerative medicine applications extend beyond current stem cell approaches. EVs could help elderly patients recover from surgery by stimulating tissue repair. They might slow neurodegenerative diseases by delivering neuroprotective factors across the blood-brain barrier. Athletes could use EVs to accelerate recovery from injuries - though this raises questions about enhancement versus healing that sports organizations will need to address.
The technology's versatility is both strength and challenge. Because EVs are involved in virtually every physiological and pathological process, the potential applications seem limitless. But translating potential into approved therapies requires solving the production, quality control, and regulatory puzzles that have stymied other biological products.
Despite explosive progress, fundamental questions remain unanswered.
We still don't fully understand the rules governing cargo selection. Why do certain proteins always get packaged into EVs while others never do? Are there master regulatory proteins we could manipulate to override normal sorting? The growing evidence for RNA-binding protein involvement is promising, but the complete mechanistic picture remains elusive.
The heterogeneity problem looms large. Even EVs from a single cell type show massive variation in size, composition, and function. Are these different vesicle populations performing distinct roles, or is it just biological noise? Should therapeutic EVs be highly purified subpopulations or heterogeneous mixtures? Different research groups reach opposite conclusions.
Long-term safety questions persist. If we suppress cancer EV production, do we also interfere with beneficial cell-to-cell communication? If we enhance regenerative EVs, could that inadvertently support tumor growth? Natural systems balance competing needs; therapeutic manipulation might have unintended consequences that appear only after years of use.
The immunological fate of therapeutic EVs needs clarification. Some studies show EVs evade immune recognition; others find robust antibody responses. The difference likely depends on production source, purification method, and administration route - but we don't yet know how to predict or control these variables.
The EV revolution challenges how we think about cells as independent units. We've long known that cells communicate through secreted molecules, but EVs reveal communication of a different order entirely.
They're not just signals - they're physical cargo transfers that can fundamentally reprogram recipient cells. A stem cell doesn't tell a damaged cell to heal; it sends EVs containing the molecular machinery to execute healing. A cancer cell doesn't broadcast a generic growth signal; it dispatches EVs with specific instructions for creating hospitable distant environments.
This represents a shift from thinking about cells as individual actors responding to environmental cues, to recognizing them as nodes in interconnected networks where EVs mediate information exchange. Your immune cells, neurons, gut bacteria, and cancer cells are all in constant conversation via these vesicular couriers.
Understanding and controlling these conversations opens therapeutic possibilities we're only beginning to explore. The same mechanism cancer exploits to spread becomes the tool we use to deliver treatments. The vesicles that trigger inflammation can be repurposed to calm it. The communication network itself becomes both target and treatment platform.
The dual nature of EVs - harmful in disease, beneficial in healing - mirrors a larger truth in biology. The same processes that enable growth and adaptation can drive pathology when dysregulated. The goal isn't to eliminate these systems but to guide them toward beneficial outcomes.
We're entering an era where medicine operates at the level of cellular communication rather than just cellular chemistry. Pills that flood the body with compounds will increasingly give way to targeted vesicles that deliver precise instructions to specific cell populations. Disease detection will shift from waiting for symptoms to monitoring molecular conversations for early warning signals.
The tiny messengers coursing through your bloodstream right now carry the future of medicine. Whether they're spreading disease or orchestrating healing depends on forces we're just learning to influence. The next decade will reveal whether we can master their language well enough to speak fluently - and rewrite the messages entirely.

Ahuna Mons on dwarf planet Ceres is the solar system's only confirmed cryovolcano in the asteroid belt - a mountain made of ice and salt that erupted relatively recently. The discovery reveals that small worlds can retain subsurface oceans and geological activity far longer than expected, expanding the range of potentially habitable environments in our solar system.

Scientists discovered 24-hour protein rhythms in cells without DNA, revealing an ancient timekeeping mechanism that predates gene-based clocks by billions of years and exists across all life.

3D-printed coral reefs are being engineered with precise surface textures, material chemistry, and geometric complexity to optimize coral larvae settlement. While early projects show promise - with some designs achieving 80x higher settlement rates - scalability, cost, and the overriding challenge of climate change remain critical obstacles.

The minimal group paradigm shows humans discriminate based on meaningless group labels - like coin flips or shirt colors - revealing that tribalism is hardwired into our brains. Understanding this automatic bias is the first step toward managing it.

In 1977, scientists discovered thriving ecosystems around underwater volcanic vents powered by chemistry, not sunlight. These alien worlds host bizarre creatures and heat-loving microbes, revolutionizing our understanding of where life can exist on Earth and beyond.

Automated systems in housing - mortgage lending, tenant screening, appraisals, and insurance - systematically discriminate against communities of color by using proxy variables like ZIP codes and credit scores that encode historical racism. While the Fair Housing Act outlawed explicit redlining decades ago, machine learning models trained on biased data reproduce the same patterns at scale. Solutions exist - algorithmic auditing, fairness-aware design, regulatory reform - but require prioritizing equ...

Cache coherence protocols like MESI and MOESI coordinate billions of operations per second to ensure data consistency across multi-core processors. Understanding these invisible hardware mechanisms helps developers write faster parallel code and avoid performance pitfalls.