Antibacterial Soap May Be Destroying Your Gut Health

TL;DR: Every night during deep sleep, your brain's glymphatic system clears toxic proteins linked to Alzheimer's. This waste clearance process depends on sleep quality, sleep stage, and even body position - making consistent, high-quality sleep essential for long-term brain health.

Every night while you sleep, your brain does something remarkable. It shrinks.
Not permanently, but just enough - about 60% - to let cerebrospinal fluid flood through channels surrounding your brain's blood vessels, washing away the day's accumulated toxic waste. This isn't poetic metaphor. It's the glymphatic system, discovered barely a decade ago, and it's forcing scientists to completely rethink the relationship between sleep and brain health.
The stakes are staggering. Your brain produces amyloid-beta proteins throughout the day - the same proteins that clump into the plaques found in Alzheimer's patients' brains. During deep sleep, your glymphatic system clears about 60% more of these toxins than when you're awake. Miss that nightly cleaning cycle, and the waste starts accumulating.
Which raises an uncomfortable question: What happens to a society that's chronically sleep-deprived?
For decades, neuroscientists had a puzzling gap in their understanding. Every organ in your body has a lymphatic system - a network of vessels that drains away cellular waste and toxins. Except the brain. Or so they thought.
In 2012, Maiken Nedergaard and her team at the University of Rochester finally found the missing piece. Using two-photon microscopy to watch fluorescent dye move through living mouse brains, they discovered a sophisticated waste clearance pathway unlike anything seen before. They called it the glymphatic system - a play on "glia," the brain cells that make it work, and "lymphatic," the system it resembles.
The glymphatic system operates primarily during deep, slow-wave sleep - when your brain waves slow to their lowest frequencies and your brain cells shrink to create space for cerebrospinal fluid to flow.
The mechanism is elegantly simple. Cerebrospinal fluid flows into the brain along channels that surround blood vessels, pushed by arterial pulsations from your heartbeat. This fluid mixes with the interstitial fluid that bathes your brain cells, picking up metabolic waste, misfolded proteins, and cellular debris. Then it drains out along venous pathways, carrying the garbage with it.
But here's the catch: this system operates primarily during sleep. And not just any sleep - specifically during deep, slow-wave sleep, when your brain waves slow to their lowest frequencies.

The reason the glymphatic system needs sleep comes down to physics and neurobiology working in concert.
During waking hours, your brain cells - neurons and glia alike - are swollen with activity. They're firing signals, consuming energy, and taking up space. The extracellular space, the gaps between cells where fluid can flow, shrinks to about 14% of total brain volume.
Then you fall asleep. Specifically, into stage 3 NREM sleep, the deepest phase of the sleep cycle. Something remarkable happens: your brain cells contract, expanding the extracellular space by roughly 60%. Suddenly, there's room for fluid to flow.
At the same time, norepinephrine - a neurotransmitter that keeps you alert when you're awake - drops to nearly zero. Every 50 seconds during deep sleep, synchronized waves of norepinephrine trigger arterial pulsations that drive cerebrospinal fluid deep into brain tissue. These rhythmic waves, coordinated with your slow brain waves, create the pressure gradients that push waste out.
The whole system depends on aquaporin-4 water channels, special proteins embedded in the feet of astrocytes - star-shaped glial cells that wrap around blood vessels. These channels regulate fluid movement with remarkable precision, ensuring waste flows in the right direction at the right speed.
Think of it as your brain's nighttime janitorial service. But instead of arriving at 9 PM when the office workers leave, this cleaning crew can only work when the building shrinks to make room for their equipment.
So what exactly is your brain flushing out during those nightly cleaning cycles?
The most significant culprit is amyloid-beta, a protein fragment that accumulates outside neurons throughout the day. In healthy brains, the glymphatic system clears amyloid-beta efficiently each night. But when clearance fails - due to poor sleep, aging, or other factors - these proteins begin aggregating into the sticky plaques that are the hallmark of Alzheimer's disease.
Tau is another major target. This protein normally helps stabilize structures inside neurons, but misfolded tau tangles are found in the brains of people with Alzheimer's and other neurodegenerative diseases. The glymphatic system helps clear both normal and abnormal tau before it can cause problems.
"Alpha-synuclein, implicated in Parkinson's disease, also gets flushed out during sleep. So do general metabolic byproducts - the molecular garbage that accumulates whenever your brain cells consume energy."
- Neuroscience Research
Recent research has found something surprising: the timing of waste clearance varies throughout the night. Different sleep stages may specialize in clearing different types of waste, though scientists are still mapping out these specifics.
What's clear is that missing even a single night of quality sleep means these toxins don't get fully cleared. And chronic sleep deprivation? That's like letting the trash pile up week after week.
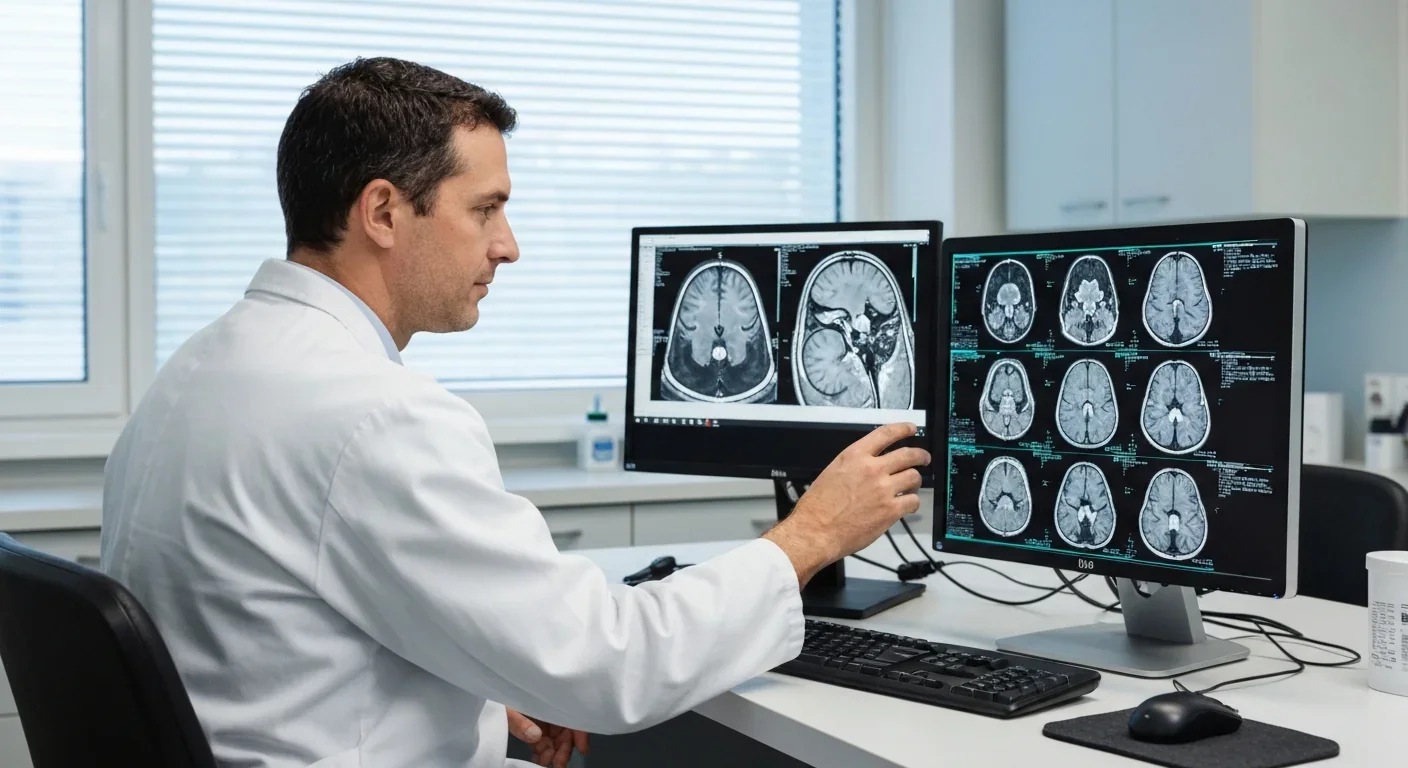
The link between sleep and Alzheimer's disease creates a vicious cycle that researchers are only beginning to understand.
Here's how it works: In the earliest stages of Alzheimer's - sometimes decades before symptoms appear - amyloid-beta begins accumulating in the brain. These protein deposits disrupt sleep architecture, particularly deep slow-wave sleep. Less deep sleep means reduced glymphatic clearance. Reduced clearance allows more amyloid to accumulate. More amyloid further disrupts sleep.
It's a self-reinforcing downward spiral. And it might explain why sleep problems are both an early warning sign of Alzheimer's and a risk factor for developing the disease.
Advanced brain imaging now allows researchers to visualize glymphatic function in living humans. Studies using the DTI-ALPS index, a specialized MRI technique, show that people with insomnia have measurably lower glymphatic activity than good sleepers. Patients with temporal lobe epilepsy show 9% lower clearance rates compared to healthy controls.
In young adults, cerebrospinal fluid movement through the brain shows a saturation ratio around 4.7%. In elderly subjects, that drops to 3.5% - a statistically significant decrease that correlates with age and increased Alzheimer's risk.
The most striking findings come from studies of age-related decline. In young adults, cerebrospinal fluid movement through the brain shows a saturation ratio around 4.7%. In elderly subjects, that drops to 3.5% - a statistically significant decrease that correlates with age.
This matters because Alzheimer's risk increases dramatically with age. If your brain's waste clearance system is already declining, you have less margin for error. Any additional factor that disrupts sleep - sleep apnea, insomnia, irregular sleep schedules - becomes more dangerous.
In 2015, researchers discovered something unexpected: how you sleep matters.
Using MRI imaging to track waste clearance in different sleeping positions, scientists found that lateral sleeping - on your side - produces significantly more efficient glymphatic flow than sleeping on your back or stomach. The exact mechanism isn't fully understood, but it appears to involve how gravity affects cerebrospinal fluid movement through the brain's drainage pathways.
This finding has interesting evolutionary implications. Humans and most other mammals naturally prefer side sleeping. Our bodies might have optimized sleep position for brain health long before we understood the reason.
The practical implications are straightforward. If you're concerned about cognitive health, sleeping on your side is a simple, zero-cost intervention that may enhance your brain's nightly detox cycle.
Some researchers are also investigating whether head position - specifically, whether your head is level, elevated, or declined - affects drainage through the cervical spine. The jury's still out, but there's emerging evidence that upper cervical alignment might influence how efficiently waste exits the brain.

Sleep disorders aren't just annoying. They're neurological emergencies unfolding in slow motion.
Take sleep apnea, a condition where breathing repeatedly stops and starts during sleep. Each apnea episode jolts you out of deep sleep into lighter stages or brief wakefulness. Your brain never gets sustained periods of slow-wave sleep, which means glymphatic clearance never reaches full efficiency.
Studies show that people with untreated sleep apnea have higher levels of amyloid-beta in their cerebrospinal fluid - exactly what you'd expect if the nightly cleaning system is failing. They also have higher rates of cognitive decline and dementia later in life.
Chronic insomnia creates similar problems through a different mechanism. People with insomnia spend less time in deep sleep even when they do sleep. Recent clinical trials using repetitive transcranial magnetic stimulation (rTMS) showed that when insomnia patients' sleep quality improved, their DTI-ALPS index - that measure of glymphatic function - increased in tandem.
"Research on Ambien found that while it helps people fall asleep, it reduced norepinephrine waves by 50%, lowering fluid transport through the brain by approximately 30%. You sleep, but your brain doesn't clean itself as effectively."
- Sleep Research Studies
Even common sleep medications can interfere with glymphatic function. Research on Ambien (zolpidem) found that while it helps people fall asleep, it reduced norepinephrine waves by 50%, lowering fluid transport through the brain by approximately 30%. You sleep, but your brain doesn't clean itself as effectively.
This creates a dilemma for the 50 to 70 million Americans with chronic sleep disorders. The treatments that help them sleep might not provide the full restorative benefits of natural, unmedicated sleep.
The good news: you're not helpless in all this.
While you can't directly control your glymphatic system - it operates during unconsciousness, after all - you can create conditions that optimize its function.
Sleep quality matters more than quantity. Seven hours of high-quality sleep with substantial deep sleep beats nine hours of fragmented, shallow sleep every time. Focus on sleep hygiene fundamentals: consistent sleep schedule, cool dark bedroom, limited screen time before bed, avoiding alcohol and caffeine in the evening.
Exercise is neuroprotective. Moderate aerobic exercise improves sleep quality, particularly slow-wave sleep. It also appears to enhance glymphatic function through mechanisms that aren't fully understood - possibly by improving cardiovascular health, which enhances the arterial pulsations that drive cerebrospinal fluid flow.
Hydration plays a role. Cerebrospinal fluid is, well, fluid. Dehydration reduces its volume and may impair circulation. While there's no evidence that drinking excessive water helps, chronic dehydration likely harms glymphatic function.
Consider sleep position. If you habitually sleep on your back or stomach, try training yourself to sleep on your side. It may take time to adjust, but the potential cognitive benefits make it worthwhile.
Treat sleep disorders aggressively. If you snore heavily, wake up gasping, or have chronic insomnia, get evaluated by a sleep specialist. Untreated sleep disorders aren't just affecting your energy - they're potentially accelerating cognitive decline.
Be strategic about sleep medications. If you need sleep aids, talk with your doctor about options that preserve sleep architecture. Newer medications like dual orexin receptor antagonists may be less disruptive to glymphatic function than older sedatives, though research is ongoing.

We're barely scratching the surface of understanding the glymphatic system.
Scientists are investigating whether the system's dysfunction contributes to other neurological conditions beyond Alzheimer's. Traumatic brain injury disrupts glymphatic clearance, which might explain why TBI increases dementia risk decades later. Multiple sclerosis, Parkinson's disease, and even glioblastoma show connections to glymphatic dysfunction.
There's growing interest in therapeutic interventions. Could we develop drugs that enhance glymphatic flow without the side effects of current sleep medications? Some researchers are exploring whether non-invasive brain stimulation techniques like rTMS could improve clearance in at-risk populations.
Others are investigating the role of the gut-brain axis. Emerging evidence suggests that the glymphatic system may clear waste not just from the brain, but also carry signaling molecules from the brain to peripheral organs, creating previously unknown communication pathways.
Advanced imaging techniques are making it possible to measure glymphatic function in individual patients, potentially identifying those at high risk for dementia before symptoms appear. Imagine a future where your doctor could order a "brain drainage scan" the way they order cholesterol tests today, catching problems early when interventions might still prevent disease.
Here's where the glymphatic story becomes a societal issue, not just a personal health concern.
Modern civilization is conducting an uncontrolled experiment on human brain health. We work jobs that demand long hours and reward sleep deprivation. We flood our evenings with blue light from screens, disrupting our circadian rhythms. We normalize poor sleep as the price of productivity.
And we're doing this precisely as our population ages. By 2050, the number of people with dementia is projected to triple globally. Some of that increase is simply more people living longer. But how much is preventable if we took sleep seriously?
Healthcare systems already struggling to care for dementia patients will face impossible burdens. The economic costs - already over $1 trillion annually in the US alone - will become unsustainable. Yet we have a low-tech, zero-cost intervention available to everyone: prioritizing sleep.
The implications ripple outward. Healthcare systems already struggling to care for dementia patients will face impossible burdens. Families will make agonizing decisions about loved ones who can't remember their names. The economic costs - already over $1 trillion annually in the US alone - will become unsustainable.
Yet we have a low-tech, zero-cost intervention available to everyone: prioritizing sleep.
This isn't about individual willpower. It's about restructuring how society values rest. School start times that align with adolescent circadian rhythms. Workplaces that respect boundaries around evening emails. Healthcare systems that screen for and treat sleep disorders as seriously as diabetes or hypertension. Urban planning that reduces noise pollution that fragments sleep.
Small changes with potentially massive long-term impacts.
The glymphatic system represents a fundamental shift in how we understand brain health. For decades, we thought of sleep as passive downtime - a biological necessity we couldn't explain but had to accept. Now we know it's an active, sophisticated maintenance process as critical as diet or exercise.
This reframing changes everything. Sleep isn't something you "get away with" skimping on. It's not a luxury for the lazy or a sign of weakness. It's mandatory biological infrastructure maintenance.
The research is clear: every night of poor sleep is a missed opportunity for your brain to clear the toxic proteins that accumulate toward dementia. Every night of quality deep sleep is an investment in your cognitive future.
You can't biohack your way around this. No supplement, nootropic, or wellness trend can substitute for the basic biological process of cerebrospinal fluid washing through your sleeping brain. The glymphatic system works on its own schedule, using mechanisms refined over millions of years of mammalian evolution.
What you can do is create the conditions for it to work optimally. Protect your sleep with the same vigilance you'd protect your diet or exercise routine. Recognize that those hours of unconsciousness aren't lost productivity - they're essential maintenance that makes all your waking hours possible.
The science gives us something rare in neuroscience: a clear, actionable pathway between behavior and brain health. We don't yet have a cure for Alzheimer's, but we increasingly understand prevention. And it starts with something beautifully simple: close your eyes, lie on your side, and let your brain clean itself.
Because tomorrow's cognitive health is being determined by tonight's sleep. The question is whether we'll take that seriously before it's too late.

Rotating detonation engines use continuous supersonic explosions to achieve 25% better fuel efficiency than conventional rockets. NASA, the Air Force, and private companies are now testing this breakthrough technology in flight, promising to dramatically reduce space launch costs and enable more ambitious missions.

Triclosan, found in many antibacterial products, is reactivated by gut bacteria and triggers inflammation, contributes to antibiotic resistance, and disrupts hormonal systems - but plain soap and water work just as effectively without the harm.

AI-powered cameras and LED systems are revolutionizing sea turtle conservation by enabling fishing nets to detect and release endangered species in real-time, achieving up to 90% bycatch reduction while maintaining profitable shrimp operations through technology that balances environmental protection with economic viability.

The pratfall effect shows that highly competent people become more likable after making small mistakes, but only if they've already proven their capability. Understanding when vulnerability helps versus hurts can transform how we connect with others.

Leafcutter ants have practiced sustainable agriculture for 50 million years, cultivating fungus crops through specialized worker castes, sophisticated waste management, and mutualistic relationships that offer lessons for human farming systems facing climate challenges.

Gig economy platforms systematically manipulate wage calculations through algorithmic time rounding, silently transferring billions from workers to corporations. While outdated labor laws permit this, European regulations and worker-led audits offer hope for transparency and fair compensation.
Quantum computers face a critical but overlooked challenge: classical control electronics must operate at 4 Kelvin to manage qubits effectively. This requirement creates engineering problems as complex as the quantum processors themselves, driving innovations in cryogenic semiconductor technology.