The Ancient Protein Clock That Ticks Without DNA

TL;DR: Red and near-infrared light can penetrate your cells and recharge mitochondria, triggering healing responses that reduce inflammation, repair tissue, and boost energy. Photobiomodulation is backed by clinical evidence for pain relief, wound healing, and vision protection, though choosing the right device and dosage is crucial for results.

What if the key to healing your body already existed in every beam of sunlight? Researchers have discovered that specific wavelengths of red and near-infrared light can penetrate your skin, reach deep into cells, and fundamentally recharge your biology at the mitochondrial level. This isn't science fiction or wellness hype. It's photobiomodulation, and it's quietly transforming how we approach pain, aging, inflammation, and cellular repair.
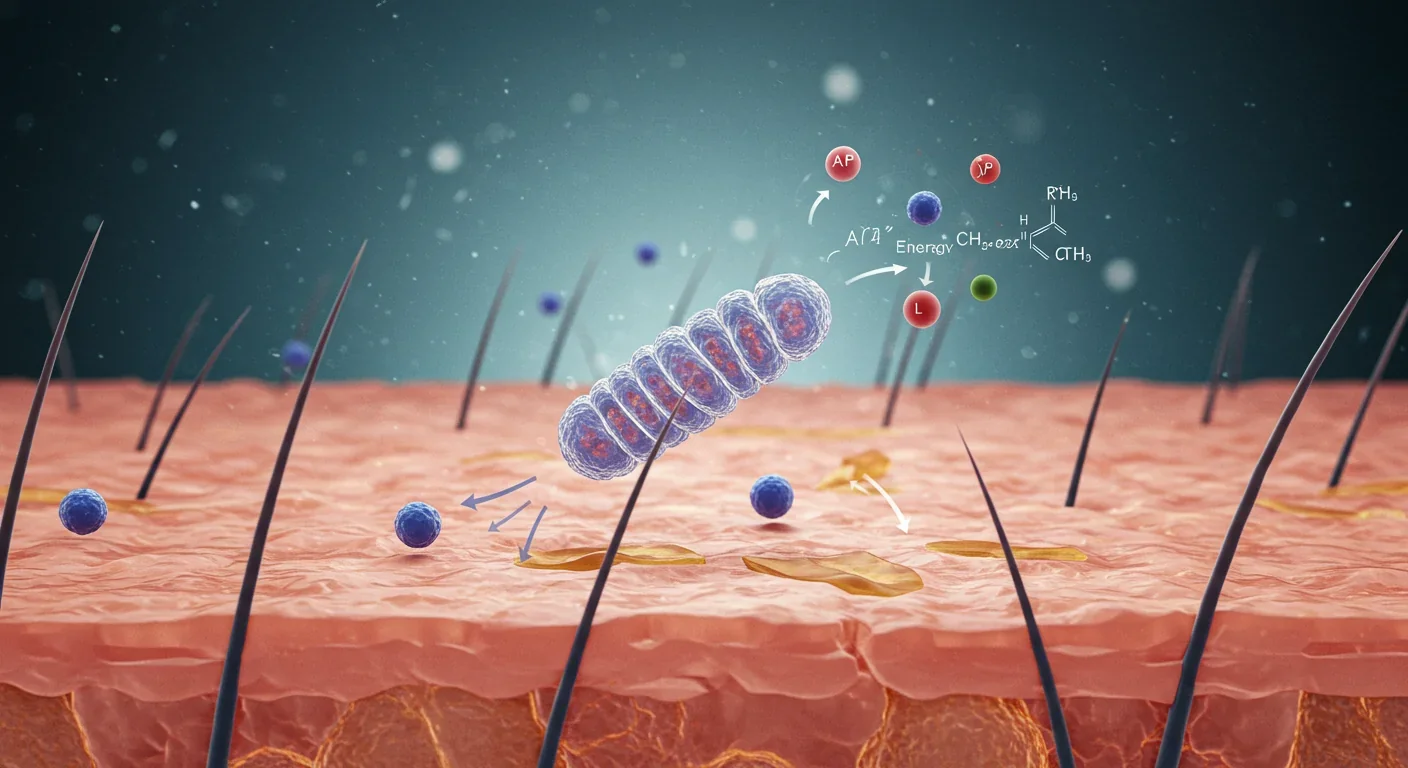
Inside every cell in your body sits a tiny power plant called a mitochondrion. These organelles generate ATP, the energy currency that keeps you alive, moving, and thinking. But what most people don't realize is that mitochondria respond to light, specifically wavelengths between 600 and 1,000 nanometers.
When red or near-infrared light hits your cells, it interacts with an enzyme called cytochrome c oxidase in the mitochondrial respiratory chain. This enzyme is like a photoreceptor buried deep in your cells. Light absorption displaces nitric oxide from cytochrome c oxidase, which normally inhibits cellular respiration. Once nitric oxide is removed, the electron transport chain accelerates, ATP production surges, and your cells get an energy boost.
But that's only the beginning. The same process triggers a cascade of secondary messengers, including reactive oxygen species (ROS), cyclic adenosine monophosphate, calcium ions, and nitric oxide itself. These molecules act as signals, telling your cells to reduce inflammation, repair tissue, modulate oxidative stress, and even protect neurons from damage.
Think of photobiomodulation as flipping a biological switch. You're not adding foreign substances or forcing unnatural reactions. You're simply using light to optimize processes your body already knows how to perform.
Photobiomodulation didn't start in a wellness spa. It began in the 1960s when Hungarian scientist Endre Mester accidentally discovered that low-level laser light stimulated hair growth in shaved mice. He called it "laser biostimulation," and it sparked decades of research into how light affects living tissue.
By the 1990s, NASA was experimenting with red LED arrays to grow plants in space and heal wounds in astronauts. The agency found that 670-nanometer red light accelerated wound healing and reduced muscle atrophy, two major concerns for long-duration spaceflight. That research laid the groundwork for modern PBM devices.
Fast forward to today, and the FDA has cleared the first photobiomodulation device for treating dry age-related macular degeneration. The Valeda Light Delivery System uses three wavelengths (590, 660, and 850 nanometers) to stimulate retinal cells and slow vision loss. Clinical trials showed patients gained an average of 2.4 letters on vision charts and had 20% less progression of geographic atrophy compared to placebo groups.
This isn't just about eyes. PBM has been studied for joint pain, traumatic brain injury, muscle recovery, skin rejuveneration, and even preventing myopia in children. The technology has moved from experimental to evidence-based, from research labs to consumer devices you can buy online.
Traditional medicine often treats symptoms with drugs or surgery. Photobiomodulation works differently because it targets the cellular environment where healing actually happens. Instead of masking pain with NSAIDs or numbing inflammation with steroids, PBM addresses the underlying metabolic dysfunction.
In studies on osteoarthritis, researchers applied near-infrared laser light at 904 to 905 nanometers to inflamed rat joints. The results were striking. Pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α dropped significantly, while anti-inflammatory marker IL-10 increased. Matrix metalloproteinases, enzymes that break down cartilage, also decreased. Pain sensitivity improved within weeks.
The mechanism is elegant. PBM modulates the NF-κB and AP-1 signaling pathways, which control inflammation at the genetic level. It also upregulates antioxidant enzymes like superoxide dismutase, helping cells neutralize damaging free radicals. You're essentially giving your cells the tools to clean up their own mess.
In the brain, photobiomodulation shows promise for neuroprotection. When neurons experience ischemia or reperfusion injury (like after a stroke), mitochondria become hyperpolarized and produce a burst of reactive oxygen species that damages cells. Researchers found that applying inhibitory infrared light at 750 or 950 nanometers during the early reperfusion window prevents this hyperpolarization. It gives mitochondria time to recover without the oxidative avalanche that usually kills neurons.
The therapeutic window is narrow, though. Applying light within 30 minutes of reperfusion maximizes neuroprotection. After 2.5 hours of treatment, cytochrome c oxidase activity returns to normal levels, and cells stabilize. Timing matters as much as dosage.
One of the most misunderstood aspects of photobiomodulation is the biphasic dose response. More light is not always better. In fact, too much light can inhibit the very processes you're trying to stimulate.
Studies consistently show a therapeutic sweet spot between 0.01 and 10 joules per square centimeter for most applications. Below that range, you don't deliver enough photons to trigger the cellular response. Above it, you risk overstimulating cells and causing inhibitory effects.
Power density also matters. Most effective protocols use 10 to 50 milliwatts per square centimeter. Sessions typically last 60 to 90 seconds per treatment area, repeated two to three times per week. It's gentle, cumulative, and relies on consistency rather than intensity.
In one rat study on joint pain, researchers compared a 50-milliwatt laser delivering 57 joules per square centimeter to a 100-milliwatt laser. The lower-power device produced better analgesia and improved range of motion. The higher power actually reduced efficacy, demonstrating the biphasic curve in action.
This is why not all red light devices are created equal. A cheap LED panel from an e-commerce site might emit the right wavelengths but deliver inconsistent power density or wrong dosages. Clinical-grade devices are calibrated to specific irradiance levels and treatment protocols. If you're considering home use, look for devices tested in peer-reviewed studies or cleared by regulatory agencies.
The clinical evidence for PBM is strongest in pain management, wound healing, and retinal health. Dermatologists use it for acne, wrinkles, and post-procedure recovery. Physical therapists apply it to strained muscles and inflamed joints. Ophthalmologists are now using it to slow macular degeneration.
In a randomized controlled trial on myopia prevention, 188 children received twice-daily PBM treatments at home. The light stimulated mitochondrial function and dopamine signaling in the retina, which helped regulate eye growth. Over 13 months, children in the treatment group showed significant axial shortening and refraction stabilization compared to controls. It's a non-pharmaceutical way to address a problem affecting millions of kids worldwide.
Athletes and biohackers have embraced PBM for muscle recovery and performance enhancement. While the research here is less robust than for medical applications, some studies suggest red and near-infrared light can reduce delayed-onset muscle soreness, increase mitochondrial density in muscle tissue, and improve endurance.
But there are limits and risks. PBM won't cure cancer, reverse Alzheimer's, or replace conventional treatments for serious diseases. Some marketers oversell the technology, making claims far beyond what the science supports. Always be skeptical of devices promising miracle cures or universal health benefits.
Safety is generally excellent when devices are used correctly. The most common side effect is mild skin warming. However, improper use, especially with high-powered lasers, can cause burns or eye damage. Never point lasers directly at your eyes unless using an FDA-cleared ophthalmic device under professional supervision.
There's also the question of individual variability. Skin tone affects light penetration. Darker skin absorbs more photons in the superficial layers, so deeper tissues receive less light. Device positioning, treatment duration, and tissue thickness all influence outcomes. What works for one person might not work for another, even with identical protocols.
If you're ready to try photobiomodulation, the first decision is whether to use a home device or seek professional treatment. Clinical settings offer higher-powered, calibrated equipment and expert guidance. Dermatology clinics, physical therapy offices, and specialized PBM centers provide targeted treatments for specific conditions.
Home devices are more convenient and cost-effective for long-term use. LED panels are the most common consumer option. They emit red (usually around 660 nanometers) and near-infrared (typically 850 nanometers) light over a wide treatment area. Panels range from small handheld units to full-body arrays.
When evaluating a device, check these specs: wavelength accuracy (look for 630-680 nm red and 810-880 nm near-infrared), power density (10-50 mW/cm² is ideal), total power output, irradiance uniformity across the panel, and third-party testing or certifications.
Avoid devices that don't publish their specifications or make vague claims about "therapeutic wavelengths." Reputable manufacturers provide detailed technical data and cite relevant studies. Some high-quality brands include Joovv, Red Therapy Co., and PlatinumLED, though many others exist.
Laser devices deliver more focused, higher-intensity light. They're useful for targeting small areas like joints, acupuncture points, or specific injuries. Class 3B and Class 4 lasers require more caution and are often restricted to professional use. Home laser devices are typically lower-powered Class 3A or 3B units.
Treatment frequency varies by goal. For acute injuries or pain, daily sessions may help. For general wellness or skin health, three to four sessions per week is common. Start conservatively, monitor your response, and adjust based on results. Most benefits accumulate over weeks to months, not days.

One ongoing debate in PBM research is whether single wavelengths or multi-wavelength combinations work better. The Valeda system, cleared by the FDA for macular degeneration, uses three wavelengths simultaneously. The logic is that different wavelengths penetrate to different depths and activate slightly different cellular pathways.
Red light around 660 nanometers penetrates about 8 to 10 millimeters into tissue, making it ideal for skin, shallow muscles, and superficial inflammation. Near-infrared at 850 nanometers reaches 30 to 40 millimeters, accessing deeper muscles, joints, and organs. A 590-nanometer yellow wavelength targets the retinal pigment epithelium specifically.
Some researchers argue that combining wavelengths provides synergistic benefits beyond what any single wavelength can achieve. Others suggest that a single, well-chosen wavelength is sufficient if dosed correctly. The evidence isn't definitive yet, but multi-wavelength devices dominate the clinical and consumer markets.
Interestingly, one study found that combining 750-nanometer and 950-nanometer wavelengths produced similar protective effects as using either wavelength alone in a neuronal injury model. This suggests redundancy in some PBM mechanisms, supporting the use of broadband near-infrared sources in consumer devices.
Perhaps the most exciting and speculative frontier for photobiomodulation is brain health. The skull presents an obvious barrier, but near-infrared light can penetrate bone and reach cortical tissue, at least to some extent.
Researchers are exploring PBM for traumatic brain injury, stroke, Alzheimer's disease, depression, and cognitive enhancement. Early results are intriguing but far from conclusive. In animal models, transcranial PBM has improved memory, reduced neuroinflammation, and protected neurons from degeneration.
Human trials are smaller and more mixed. Some studies show cognitive improvements or mood benefits, while others find no effect. The challenge is delivering a therapeutic dose to deep brain structures without overheating the scalp or using impractically high power levels.
Intranasal PBM is an alternative approach. Devices deliver light through the nasal cavity, where the cribriform plate (a thin bone separating the nose from the brain) allows better photon penetration. A few small studies suggest benefits for anxiety, sleep, and focus, but larger, rigorous trials are needed.
Neuromodulation through light is biologically plausible. Mitochondrial dysfunction plays a role in many neurodegenerative diseases. If PBM can boost neuronal energy metabolism, reduce oxidative stress, and modulate inflammation, it could theoretically slow or prevent brain aging. We're just not there yet with the evidence.
The field of photobiomodulation is young, dynamic, and sometimes controversial. While dozens of conditions have shown positive responses in preliminary studies, many lack the large-scale, randomized controlled trials that define modern medicine's gold standard.
Funding is a challenge. Because light can't be patented the way drugs can, pharmaceutical companies have little incentive to invest in PBM research. Most studies are small, conducted by academic labs or device manufacturers. This creates potential conflicts of interest and limits the scope of what gets studied.
Standardization is another hurdle. Protocols vary wildly across studies, making comparisons difficult. One trial might use 10 milliwatts per square centimeter for five minutes, while another uses 50 milliwatts for 90 seconds. Different wavelengths, devices, treatment frequencies, and patient populations further complicate the evidence base.
Despite these challenges, the trend is toward more rigorous science. Large institutions like Harvard Medical School, the National Institutes of Health, and the Department of Defense are funding PBM research, particularly for brain injury and chronic pain. As devices improve and protocols standardize, the quality of evidence should rise.
One promising area is personalized PBM. Genetic variations in cytochrome c oxidase and other photoreceptors may influence individual responses to light therapy. Wearable sensors could eventually monitor tissue oxygen levels or mitochondrial function in real time, adjusting light dose dynamically for optimal results.
Another frontier is combining PBM with other therapies. Researchers are testing light plus exercise, light plus electrical stimulation, and light plus pharmacological agents to see if multimodal approaches amplify benefits.
If you're curious about photobiomodulation, start with clear goals and realistic expectations. Define what you want to improve: joint pain, skin texture, muscle recovery, sleep, or something else. Research the evidence for that specific application. Not all uses are equally supported by science.
Consult a healthcare provider, especially if you have a medical condition or take medications. While PBM is generally safe, it's not appropriate for everyone. Pregnant women, people with photosensitivity disorders, and those with active cancer should avoid it or use it only under medical supervision.
If you choose a home device, begin with short sessions (5 to 10 minutes) and gradually increase as tolerated. Position the device 6 to 12 inches from your skin for LED panels, or follow manufacturer guidelines for handheld units. Consistency beats intensity, every session counts more than any single high-dose exposure.
Track your results. Keep a journal noting treatment times, settings, and any changes in symptoms or well-being. This helps you identify what works and adjust your protocol. Remember, benefits accumulate slowly. Give it at least four to six weeks before deciding whether it's effective.
For professional treatments, seek providers who understand dosimetry and have experience with PBM. Ask about their device specifications, treatment protocols, and evidence base. Beware of anyone promising guaranteed cures or using pseudoscientific jargon.
Photobiomodulation represents a shift in how we think about health interventions. Instead of adding chemicals or cutting tissue, we're using energy to optimize biology. It's non-invasive, has minimal side effects, and taps into mechanisms that evolution already built into our cells.
The science is still catching up to the hype, but the core principles are sound. Light interacts with mitochondria. Mitochondria drive cellular energy and signaling. Better mitochondrial function supports healing, reduces inflammation, and may slow aging. The rest is about refining protocols, identifying the best applications, and understanding individual variability.
As research expands and technology improves, PBM could become a routine tool in medicine, athletics, and personal wellness. Imagine a future where light therapy is as common as taking vitamins or going to the gym. Where recovering from surgery includes targeted photobiomodulation to speed tissue repair. Where preventing vision loss means sitting in front of a light device for 90 seconds a day.
We're not quite there yet, but we're getting closer. The science is advancing, the devices are improving, and the evidence base is growing. Whether photobiomodulation becomes a mainstream therapy or remains a niche treatment depends on the next decade of research and clinical validation.
For now, the light is shining, and the cells are listening. What you do with that knowledge is up to you.

Ahuna Mons on dwarf planet Ceres is the solar system's only confirmed cryovolcano in the asteroid belt - a mountain made of ice and salt that erupted relatively recently. The discovery reveals that small worlds can retain subsurface oceans and geological activity far longer than expected, expanding the range of potentially habitable environments in our solar system.

Scientists discovered 24-hour protein rhythms in cells without DNA, revealing an ancient timekeeping mechanism that predates gene-based clocks by billions of years and exists across all life.

3D-printed coral reefs are being engineered with precise surface textures, material chemistry, and geometric complexity to optimize coral larvae settlement. While early projects show promise - with some designs achieving 80x higher settlement rates - scalability, cost, and the overriding challenge of climate change remain critical obstacles.

The minimal group paradigm shows humans discriminate based on meaningless group labels - like coin flips or shirt colors - revealing that tribalism is hardwired into our brains. Understanding this automatic bias is the first step toward managing it.

In 1977, scientists discovered thriving ecosystems around underwater volcanic vents powered by chemistry, not sunlight. These alien worlds host bizarre creatures and heat-loving microbes, revolutionizing our understanding of where life can exist on Earth and beyond.

Automated systems in housing - mortgage lending, tenant screening, appraisals, and insurance - systematically discriminate against communities of color by using proxy variables like ZIP codes and credit scores that encode historical racism. While the Fair Housing Act outlawed explicit redlining decades ago, machine learning models trained on biased data reproduce the same patterns at scale. Solutions exist - algorithmic auditing, fairness-aware design, regulatory reform - but require prioritizing equ...

Cache coherence protocols like MESI and MOESI coordinate billions of operations per second to ensure data consistency across multi-core processors. Understanding these invisible hardware mechanisms helps developers write faster parallel code and avoid performance pitfalls.